Eye drop recall
Web 21 hours agoHealth Manufacturer recalls eyedrops after possible link to bacterial infections Per the CDCs latest update 68 patients across 16 states have been infected with Pseudomonas aeruginosa. Web The Food and Drug Administration FDA has recently recalled three brands of eye drops including one that has been linked to serious infections vision loss and a death.
:max_bytes(150000):strip_icc()/ezricare-e226fc86d3ab4331a7863d5ccee0e584.png)
Recalled Eye Drops Linked To Infections Vision Loss
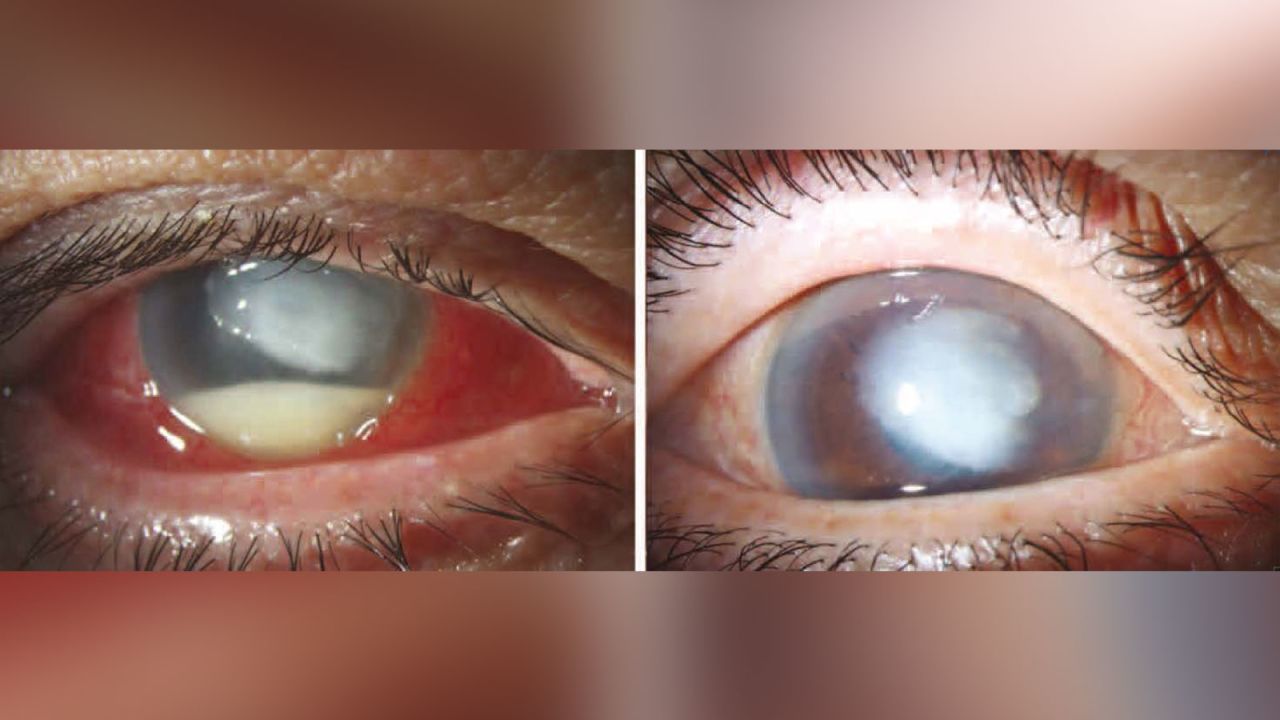
Web An alarming outbreak of extensively drug-resistant bacteria linked to eye drops has now sickened 68 people across 16 states according to the latest update from the Centers for Disease Control.

. Web WASHINGTON US. Web Bacteria in recalled eye drops linked to cases of vision loss surgical removal of eyeballs Global Pharma Healthcare recalled its Artificial Tears Lubricant Eye Drops that were. The two lots were pulled because of problems that could result in blindness the company said.
Web At least four eyedrops have been recalled recently. Both companies said the recalls were conducted in consultation with the FDA. Web CNN Global Pharma Healthcare is issuing a recall of its Artificial Tears Lubricant Eye Drops that were distributed by EzriCare and Delsam Pharma due to possible contamination the US Food.
Web Two types of artificial tears eye drops have been voluntarily recalled following 55 reports of adverse use effects including eye infections vision loss and even a bloodstream infection that led. UC Davis Health experts share what you need to know. They include EzriCare and Delsam Pharmas Artificial Tears which have been associated with the bacterial infection.
Two other products. Health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and serious injury. Web Pharmedica is recalling its Purely Soothing 15 MSM Drops meant to treat eye irritation.
Consumers are advised to stop using the following brands and return them to the place of purchase. Web The Food and Drug Administration posted separate recall notices for certain eyedrops distributed by Pharmedica and Apotex after the companies said they are voluntarily pulling several lots of their products from the market.

Eye Drops Recalled By Two Companies Over Safety Concerns Wsj
Global Pharma Healthcare Issues Voluntary Nationwide Recall Of Artificial Tears Lubricant Eye Drops Due To Possible Contamination Fda

F57pm3jlz5eg9m

South Florida Medical Company Recalls Eye Drops For Glaucoma Miami Herald

What To Know About The Recent Eye Drop Recalls The New York Times

O N2vpc3liz1vm
:max_bytes(150000):strip_icc()/8-best-eye-drops-for-allergies-of-2023-tout-1bfb54eb47dc4b30930c5666601e0cac.jpg)
Recalled Eye Drops Linked To Infections Vision Loss

Fda Two More Eyedrop Brands Recalled Due To Risks Abc News
9qh5htchxgadam

Ezricare Eye Drops Recall Linked To Infections Blindness Death

Eye Drop Recall Everything You Need To Know Right Now

N93sarakovp4ym
Eye Drop Recall Walmart Cvs Products May Not Be Sterile
02mf3hh9wahuam

Eye Drops Recalls 2023 Products Recalled Because They May Not Be Sterile

Fda Eye Drops Recall Lawyer Contaminated Artificial Tears Attorney Hastings Law Firm Medical Malpractice Lawyers

Eye Infections From Tainted Eyedrops May Be More Widespread Doctors Worry